Learn about the chemical structure HCOOCH CH2 H2O, which involves esters, alcohols, and hydration reactions. Discover the significance, applications, and reactions behind this chemical formula in organic chemistry.
Introduction: What is HCOOCH CH2 H2O?
The chemical system HCOOCH CH2 H2O is a aggregate of three essential chemicals, regarding an ester, an alcohol, and water. To ruin it down, it includes HCOOCH (a methyl ester), CH2 (a part of a carbon chain, doubtlessly indicating the involvement of an alcohol), and H2O (water). This article will discover the chemical shape, homes, reactions, and programs of this method in numerous scientific contexts.
Understanding this molecule calls for a fundamental knowledge of natural chemistry, mainly esters, alcohols, and hydration reactions. These components are large in several chemical reactions, from growing fragrances and flavors to business processes. In this text, we will dive deeper into each element and explain how they arrive together in reactions, highlighting their importance within the chemistry global.
What is an Ester?
An ester is a chemical compound shaped from the reaction among an acid and an alcohol, normally by means of a process called esterification. Esters are widely used in the production of fragrances, flavors, and as solvents in commercial applications. They are often characterized by the method RCOOR’, wherein R and R’ constitute organic corporations.
In the case of HCOOCH, we’re searching at a methyl ester of formic acid. Formic acid, or HCOOH, reacts with methanol (CH3OH) to shape HCOOCH (methyl formate), an ester. This ester is generally utilized in commercial tactics, particularly as a solvent, in fragrances, and as a reagent in chemical synthesis.
Formation of Esters
Esters are generally fashioned by way of a response between an alcohol and an acid within the presence of heat and/or a catalyst. For example:
Formic acid (HCOOH) + Methanol (CH3OH) → Methyl formate (HCOOCH) + Water (H2O)
This reaction is called esterification. The ester HCOOCH produced right here performs a important position in lots of chemical tactics.
The Role of Alcohol in HCOOCH CH2 H2O
The second part of the formula, CH2, suggests a connection to an alcohol institution. In many esterification reactions, alcohols play an essential position in breaking down acids to create esters. Methanol (CH3OH) and ethanol (C2H5OH) are two typically used alcohols in ester formation, although CH2 within the formulation could advocate a methylene institution or CH2OH (a hydroxymethyl institution), relying on the unique chemical shape being described.
In the context of HCOOCH CH2, it’s possible that the CH2 refers to part of a molecule that connects an alcohol to a carbonyl institution, where the alcohol’s hydroxyl group (-OH) is worried in a reaction. The alcohol forms an ester by using bonding with the acid element and freeing a molecule of water inside the technique.
Hydration Reactions: The Role of Water (H2O)
In the formulation HCOOCH CH2 H2O, H2O (water) is a vital player in many chemical reactions, specially in esterification and hydrolysis. During esterification, water is often a byproduct. In a few cases, water may be used to reverse the esterification procedure, breaking the ester again into the original alcohol and acid — that is referred to as hydrolysis.
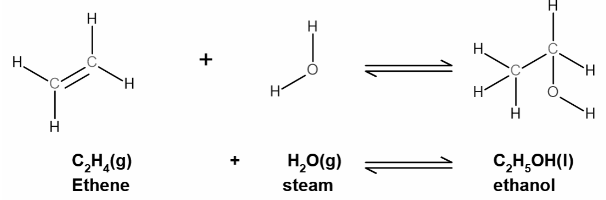
In the case of HCOOCH CH2 H2O, it’s possible that water performs a role in facilitating the response or influencing the equilibrium between ester and acid. When an ester undergoes hydrolysis, the following response happens:
Ester (RCOOR’) + H2O → Alcohol (ROH) + Acid (RCOOH)
This is a reversible reaction, and depending at the conditions (along with temperature or catalysts), water can both power the formation of the ester or break it down.
Applications of Esters in Real-World Chemistry
Esters like HCOOCH are extremely vital in numerous industries. Let’s study a few realistic programs:
- Fragrance and Flavor Industry: Esters are extensively used to create fragrances and flavors because of their first-rate, fruity odors. Methyl formate (HCOOCH) itself has a sweet, fruity scent, making it useful in flavoring sellers for goodies, drinks, and perfumes. This precise ester may play a position in more complicated fragrances inside the perfume industry.
- Solvents and Industrial Use: Esters along with methyl formate are used as solvents in numerous chemical methods. They are regularly employed in cleaning sellers, adhesives, paints, and coatings. Their ability to dissolve a extensive variety of substances whilst being extraordinarily non-poisonous makes them a favored choice for many industrial programs.
- Biochemical Reactions: Esters are crucial in biochemistry and pharmacology, wherein they take part in numerous enzyme-catalyzed reactions. The hydrolysis of esters is a vital step inside the breakdown of sure biomolecules in dwelling organisms. Understanding how esters engage with water and enzymes is critical for drug improvement and metabolic studies.
Key Reactions Involving HCOOCH CH2 H2O
- Esterification Reactions: Esterification is a essential response that forms esters. It occurs whilst an acid (like formic acid) reacts with an alcohol (like methanol) inside the presence of heat. The result is an ester (HCOOCH) and water (H2O). This procedure may be used to produce a huge style of esters for commercial and business use.
- Hydrolysis of Esters: When water is added to an ester, it can ruin the ester lower back into its discern alcohol and acid. This system, referred to as hydrolysis, is normally visible in both chemical labs and organic systems. For instance:
HCOOCH + H2O → HCOOH + CH3OH
This is a basic acid-catalyzed hydrolysis technique. In commercial settings, hydrolysis reactions can be reversed or controlled to achieve either the alcohol or acid in its pure shape.
- Transesterification Reaction: In biodiesel manufacturing, transesterification involves converting triglycerides (fat) into biodiesel via reacting them with methanol or ethanol. This is a key method in biofuel production, and esters are vital to the reaction mechanism.
How HCOOCH CH2 H2O is Important in Organic Chemistry
The molecular structure HCOOCH CH2 H2O represents a typical esterification response in which an alcohol, acid, and water are involved. In natural chemistry, information the reactivity of esters, alcohols, and acids is critical for:
- Synthesis of complex natural compounds
- Drug system: Many prescribed drugs contain ester bonds.
- Biochemical tactics: Hydrolysis and esterification play critical roles in cell metabolism.
The presence of water in ester-related reactions indicates the equilibrium dynamics among the ester and its original components (acid and alcohol). The chemical interactions in this context are critical for designing reactions in both laboratory and commercial settings.
Conclusion: The Importance of HCOOCH CH2 H2O in Chemistry and Beyond
The formula HCOOCH CH2 H2O embodies key concepts in natural chemistry, involving esters, alcohols, and water. Esters are imperative to severa chemical tactics, from business programs to biological features, and the aggregate of HCOOCH (methyl formate), CH2, and H2O demonstrates how these additives interact in esterification, hydrolysis, and other associated reactions.
Understanding those chemical reactions is not simplest vital for scientists and researchers however additionally for industries counting on esters for products such as fragrances, solvents, and biofuels. The capability to govern ester formation and hydrolysis reactions opens up avenues for innovations in prescription drugs, meals chemistry, green electricity, and biochemical engineering.
By delving into the chemistry in the back of HCOOCH CH2 H2O, we gain a deeper appreciation for the flexibility of organic molecules and their essential roles in both nature and generation. Whether you’re reading for an examination or exploring capacity industrial programs, this newsletter gives treasured insights into the significance of ester and hydration reactions, in the end contributing to a extra comprehensive knowledge of organic chemistry.
FAQs About HCOOCH CH2 H2O
What is the importance of the formula HCOOCH CH2 H2O?
The formula HCOOCH CH2 H2O represents a mixture of ester and alcohol reactions, often visible within the formation of esters and their next hydrolysis. It shows a chemical system wherein methyl formate (HCOOCH) and water (H2O) are involved in reactions which include esterification or hydrolysis.
What is esterification and how does it relate to HCOOCH CH2 H2O?
Esterification is the method wherein an alcohol and an acid react to form an ester and water. In the case of HCOOCH, the response happens among formic acid and methanol. The response produces methyl formate (HCOOCH) and releases water (H2O).
Can HCOOCH CH2 H2O be utilized in biofuel production?
Yes, esters like HCOOCH are imperative to the transesterification manner in biodiesel manufacturing, in which fat or oils react with alcohols to form biodiesel and glycerol. Water performs a critical role in controlling the response in biodiesel synthesis.
What are the commercial programs of methyl formate (HCOOCH)?
Methyl formate is broadly utilized in industries as a solvent, inside the production of fragrances, and inside the chemical synthesis of different compounds. Its capability to dissolve numerous materials and its fruity scent make it beneficial in flavors and fragrances.
How does hydrolysis affect esters?
Hydrolysis is the technique of breaking down esters the usage of water. It reverses the esterification procedure, producing an alcohol and an acid. This is an important process in each business programs and biological structures, wherein enzymes frequently catalyze ester hydrolysis for metabolic capabilities.
How does HCOOCH CH2 H2O relate to biological processes?
In biological structures, the hydrolysis of esters plays a extensive role in digestion and metabolism. Enzymes like lipases and esterases assist wreck down ester bonds in fat and oils, converting them into fatty acids and alcohols, which the body can use for power. This system is essential inside the breakdown of lipids, as well as within the absorption of vitamins which might be fats-soluble.
Can water be used to opposite esterification reactions?
Yes, in esterification reactions, water can act as a hydrolyzing agent, reversing the esterification process. By adding water to an ester, the reaction can shift to provide the original alcohol and acid. This is beneficial in both laboratory synthesis and in natural approaches such as digestion and metabolic breakdown of compounds.